Post by petea on May 5, 2024 19:21:02 GMT
As I am sure I have mentioned elsewhere, I do have a number of microscopes. I guess that is not surprising as I'm a microbiologist, but while we have quite a few in my company that are used for real work, I also have a collection of older and interesting models either at home, or in the studio in Germany. I also have a large collection of historic microscope slides and have been collecting, mainly Victorian, prepared slides of diatoms for many years. Now these organisms have wonderful geometric 'skeletons', but being made of silica are rather hard to visualise under the microscope. One can use a number of contrast enhancing methods to view them and the best of these is differential interference contrast (DIC).
I have three microscopes in my collection fitted with DIC, two are inverted (one for use mainly with live, biological specimens, a Reichert Biovert, and a second really aimed at materials, the Reicgert MeF2). I also have a large research microscope with most of the accessories which has DIC, but which still lives at work (actually there is a Zeiss Universal there too for which I have DIC, but it is not operational at the moment). Despite this, I have always fancied a Nikon fitted with DIC and have been keeping my eye out for one that is not too insanely expensive. A couple of weeks back I spotted a full set of DIC optics for sale in Spain and purchased them and they are on their way to my address in Germany (thus avoiding the import duty they would attract if delivered to the UK). They fit the Nikon Optiphot, a smallish, modular research microscope from the 1980s. These are pretty robust and many can still commonly be found in use in companies and universities today: we have one (and a related model) in our laboratory and they are used very day.
As the Otiphots at work were in use I looked around for one I could use to host the DIC optics I had bought and spotted one on a university equipment resellers website. It looked to be cosmetically very nice and was a very good price, and they included photos showing an image taken through the microscope. However, I assumed there was a good reason that the university were disposing of it. When it arrived it was immediately clear why as the condenser rack was right at the bottom of its travel and completely stuck. A few other controls, such as the lamp adjuster, were stiff and the optics needed a bit of a clean. So, I set to and checked to see what the problem was and hoped the rack and pinion had not been damaged. After dismantling the condenser rack assembly it was clear that it had been driven off its rack and whoever did it was not able to realign it and just got it stuck, not aided by the grease having hardened. It took an hour or so to clean, re-lubricate and reassemble it and now it works perfectly. I also cleaned the microscope externally as well as the optics and stripped and lubricated the phase-contrast condenser it came with. It worked well and I aligned all of the optics correctly and I checked it out using a random selection of slides from my collection.
Although it produced a bright and uniformly lit image, I could see there was dust on the primary mirror in the base and so today I stripped the base from the stand and looked. There was some dust, but most of the 'clouding' was caused by fine oxidation of the surface mirroring which sounds and looks worse that it is, but I still wanted to clean it. Microscope mirrors, like those in reflecting telescopes, are mirrored on their surface and not on the back of a glass support which makes cleaning them much more difficult. While a telescope mirror often has a hardened layer applied to the mirroring due them being exposed to the elements more, microscope mirrors are not, and the (usually) vacuum deposited aluminium is unprotected. This leads to it developing slight oxidation over time. Using a large number of optical tissues I carefully removed the layer, but had to work through the aperture in the mirror block as I did not want to remove it as the light path would then have needed recollimating (which although it is done mechanically rather than optically, I do not have the special tool for and didn't fancy making one). Anyway, the cleaning worked perfectly and the microscope is up and running and ready for the DIC optics when they arrive. I also have an episcopic illumination module for it and a fluorescence module and a wide range of objectives, but at the moment I only have it set up for transmitted, normal light.
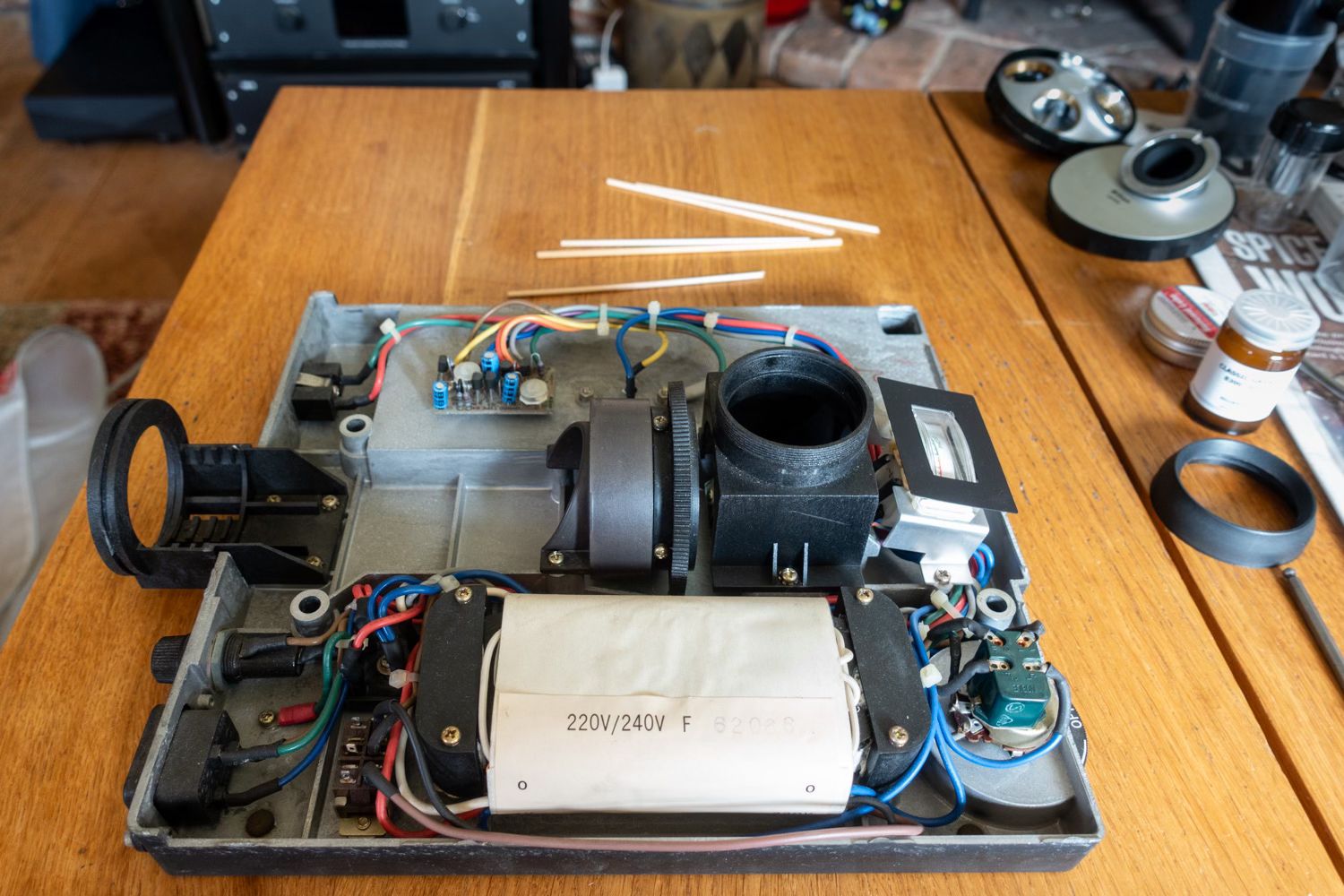
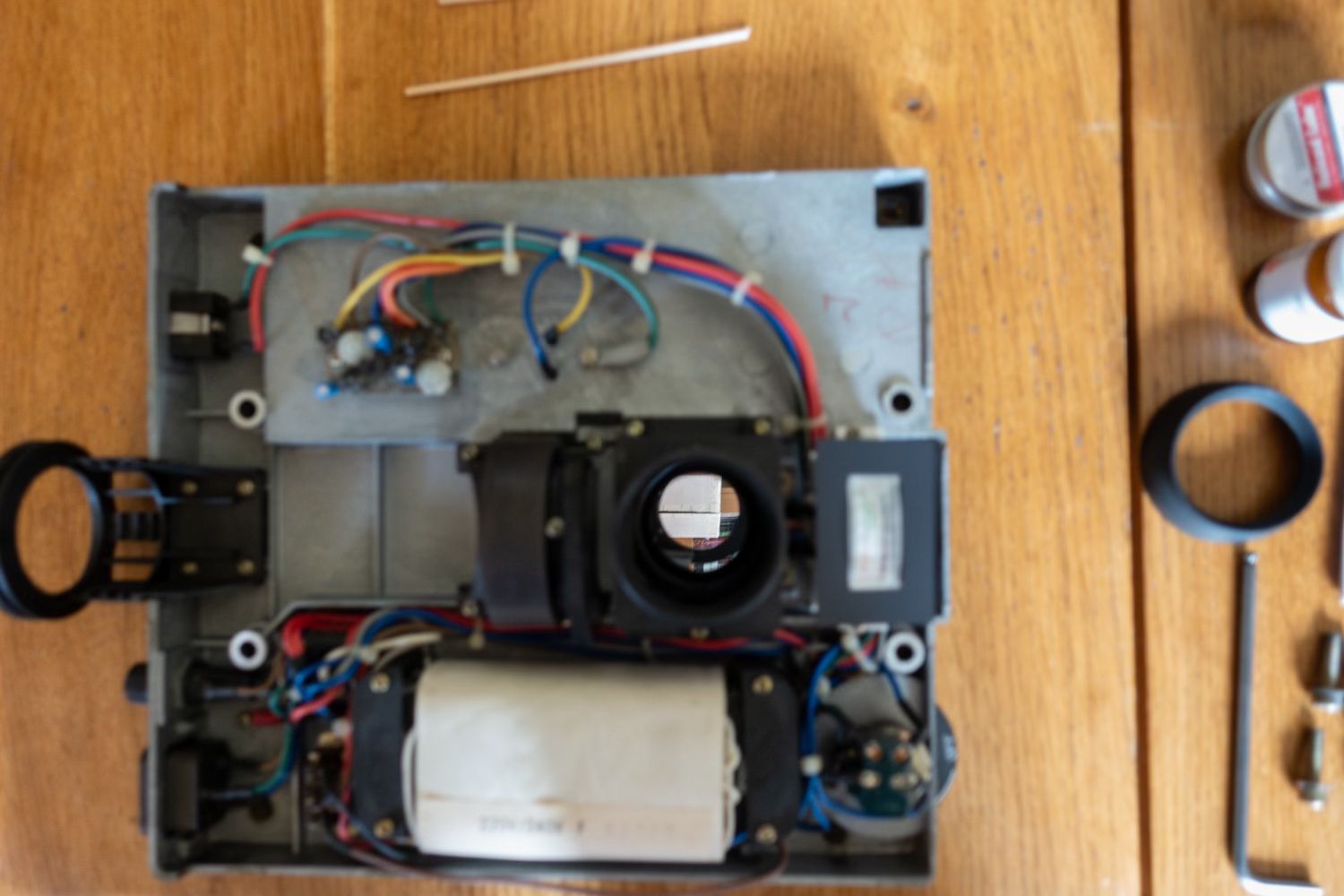
Here is a view of the base with primary mirror box and another showing the nice shiny mirror after cleaning.
Here is the microscope fully reassembled (every home should have one of the coffee table I reckon!).

I then stuck an 18MP CMOS camera on top and took a few pictures of a couple of old slides. This shows a comparison of the 3 lighting system available at the moment (using a 10X objective).
Bright-field (ie the standard lighting for a light microscope). This is a marine diatom, called Actinoptychus stella. It is about 115 µm in diameter and has been mounted without the cell contents being oxidised and still retains some pigmentation even after about 80-odd years.

Dark field illumination. This results in the subject being lit against a dark ground (by using a patch plate in the condenser that prevents direct transmission) and in this case worked well despite it being an old slide (I only removed 3 dust specks that were embedded in the mountant along with specimen in 'post production).

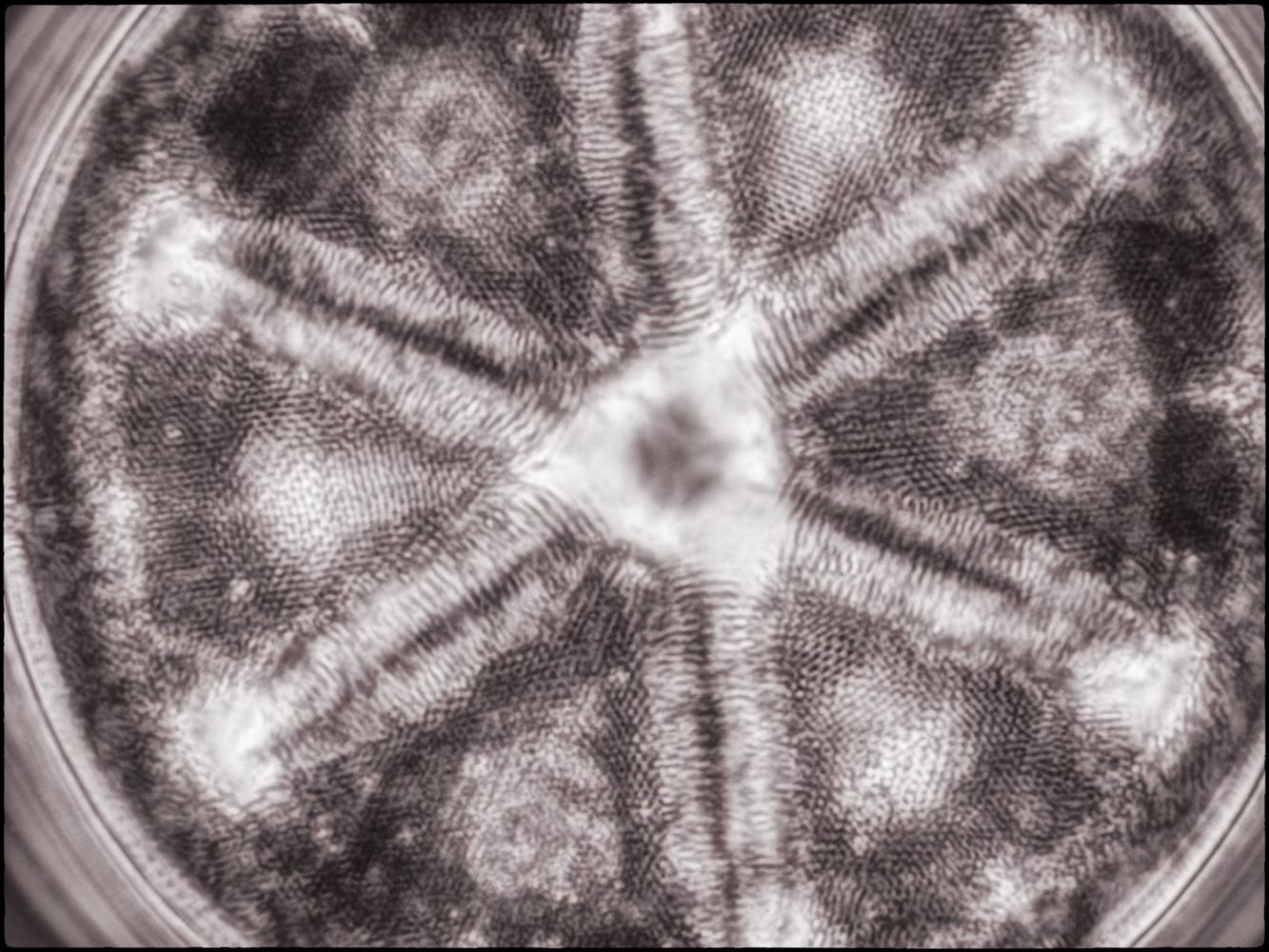
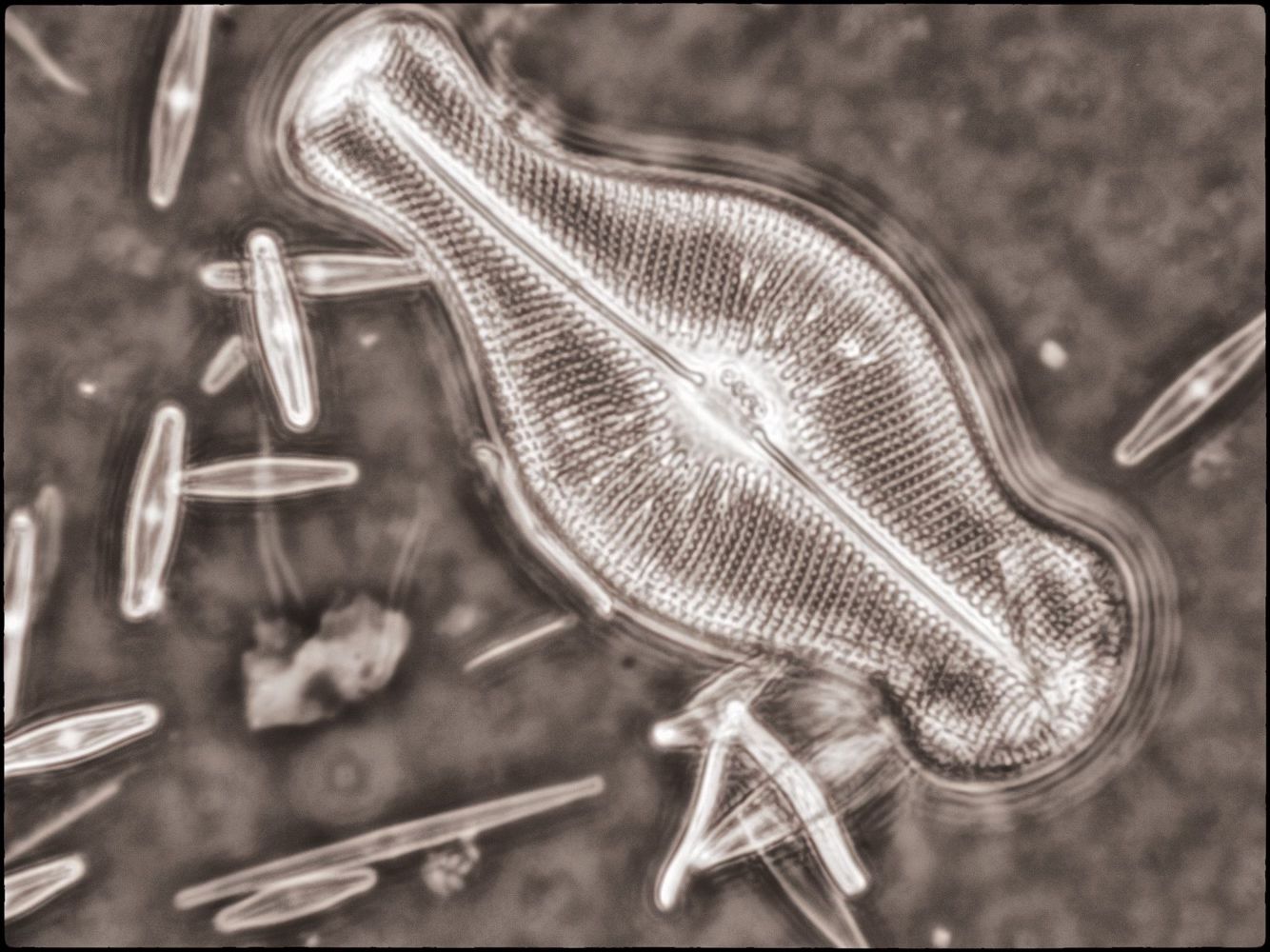
Phase Contrast (which uses phase displaced light to create positive and negative interference in areas of greater optical density in the object, and in the mountant and on the surface of the slide!).

I also created a couple of image stacks at a higher magnification (using a 40X objective) of this and another slide using phase contrast. I rendered them in monochrome and then toned them digitally in the final edit (stacking using Helicon Focus and editing using Adobe Lightroom and Nik ColorEffex 6 and Output Sharpener).
I might set up the episcopic lighting tomorrow, but will save you from that!
I have three microscopes in my collection fitted with DIC, two are inverted (one for use mainly with live, biological specimens, a Reichert Biovert, and a second really aimed at materials, the Reicgert MeF2). I also have a large research microscope with most of the accessories which has DIC, but which still lives at work (actually there is a Zeiss Universal there too for which I have DIC, but it is not operational at the moment). Despite this, I have always fancied a Nikon fitted with DIC and have been keeping my eye out for one that is not too insanely expensive. A couple of weeks back I spotted a full set of DIC optics for sale in Spain and purchased them and they are on their way to my address in Germany (thus avoiding the import duty they would attract if delivered to the UK). They fit the Nikon Optiphot, a smallish, modular research microscope from the 1980s. These are pretty robust and many can still commonly be found in use in companies and universities today: we have one (and a related model) in our laboratory and they are used very day.
As the Otiphots at work were in use I looked around for one I could use to host the DIC optics I had bought and spotted one on a university equipment resellers website. It looked to be cosmetically very nice and was a very good price, and they included photos showing an image taken through the microscope. However, I assumed there was a good reason that the university were disposing of it. When it arrived it was immediately clear why as the condenser rack was right at the bottom of its travel and completely stuck. A few other controls, such as the lamp adjuster, were stiff and the optics needed a bit of a clean. So, I set to and checked to see what the problem was and hoped the rack and pinion had not been damaged. After dismantling the condenser rack assembly it was clear that it had been driven off its rack and whoever did it was not able to realign it and just got it stuck, not aided by the grease having hardened. It took an hour or so to clean, re-lubricate and reassemble it and now it works perfectly. I also cleaned the microscope externally as well as the optics and stripped and lubricated the phase-contrast condenser it came with. It worked well and I aligned all of the optics correctly and I checked it out using a random selection of slides from my collection.
Although it produced a bright and uniformly lit image, I could see there was dust on the primary mirror in the base and so today I stripped the base from the stand and looked. There was some dust, but most of the 'clouding' was caused by fine oxidation of the surface mirroring which sounds and looks worse that it is, but I still wanted to clean it. Microscope mirrors, like those in reflecting telescopes, are mirrored on their surface and not on the back of a glass support which makes cleaning them much more difficult. While a telescope mirror often has a hardened layer applied to the mirroring due them being exposed to the elements more, microscope mirrors are not, and the (usually) vacuum deposited aluminium is unprotected. This leads to it developing slight oxidation over time. Using a large number of optical tissues I carefully removed the layer, but had to work through the aperture in the mirror block as I did not want to remove it as the light path would then have needed recollimating (which although it is done mechanically rather than optically, I do not have the special tool for and didn't fancy making one). Anyway, the cleaning worked perfectly and the microscope is up and running and ready for the DIC optics when they arrive. I also have an episcopic illumination module for it and a fluorescence module and a wide range of objectives, but at the moment I only have it set up for transmitted, normal light.
Here is a view of the base with primary mirror box and another showing the nice shiny mirror after cleaning.


Here is the microscope fully reassembled (every home should have one of the coffee table I reckon!).

I then stuck an 18MP CMOS camera on top and took a few pictures of a couple of old slides. This shows a comparison of the 3 lighting system available at the moment (using a 10X objective).
Bright-field (ie the standard lighting for a light microscope). This is a marine diatom, called Actinoptychus stella. It is about 115 µm in diameter and has been mounted without the cell contents being oxidised and still retains some pigmentation even after about 80-odd years.

Dark field illumination. This results in the subject being lit against a dark ground (by using a patch plate in the condenser that prevents direct transmission) and in this case worked well despite it being an old slide (I only removed 3 dust specks that were embedded in the mountant along with specimen in 'post production).

Phase Contrast (which uses phase displaced light to create positive and negative interference in areas of greater optical density in the object, and in the mountant and on the surface of the slide!).

I also created a couple of image stacks at a higher magnification (using a 40X objective) of this and another slide using phase contrast. I rendered them in monochrome and then toned them digitally in the final edit (stacking using Helicon Focus and editing using Adobe Lightroom and Nik ColorEffex 6 and Output Sharpener).


I might set up the episcopic lighting tomorrow, but will save you from that!







 📶NAS > Gustard x26p DAC > Benchmark LA4 Pre-Amp > NAD M22-V2 Amplifier > Revel F228Be Speakers
📶NAS > Gustard x26p DAC > Benchmark LA4 Pre-Amp > NAD M22-V2 Amplifier > Revel F228Be Speakers